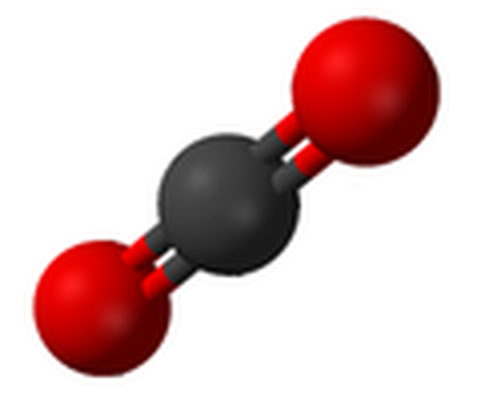
Structure and Bonding of Dry Ice
Carbon is the chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent, making four electrons available to form covalent chemical bonds. All forms of carbon are highly stable, requiring high temperature to react with oxygen. The most common oxidation state of carbon, in inorganic compounds, is +4, while +2 is found in carbon monoxide.
The electronic configuration of the carbon atom, allows it to form a series of hybridized atomic orbitals via hybridization. The carbon atoms in the elemental substances diamond, graphite and buckyballs (Buckminsterfullerenes) bond together covalently by sharing pairs of electrons (a covalent bond is formed between two atoms when their orbitals overlap and share a pair of electrons. When the orbitals overlap along an axis between the atoms (internuclear axis), they form a sigma bond. In this type of bonding the electron density is highest in the space between the atoms). These covalent bonds have directional properties. This in turn gives carbon the ability to adapt to different molecular and crystal structures. The nature of these bonds outline different physical and chemical properties of carbon allotropes.
Carbon, like most first-row elements in the Periodic Table, has atomic orbitals that can be hybridized (blended). This is due to the fact that the s-orbital and p-orbitals of carbon's second electronic shell energies are very similar. As a result, carbon can be adapted to form chemical bonds in different geometries.
Since carbon dioxide is a covalently bonded molecule it is subjected to three forces that can operate between covalent molecules:
Carbon dioxide does not bond with hydrogen (hence the formula CO2) so it is not affected by hydrogen bonds nor is it affected by dipole-dipole interactions because carbon dioxide is a non-polar molecule. So the only intermolecular force that affects it is London dispersion forces:
The electronic configuration of the carbon atom, allows it to form a series of hybridized atomic orbitals via hybridization. The carbon atoms in the elemental substances diamond, graphite and buckyballs (Buckminsterfullerenes) bond together covalently by sharing pairs of electrons (a covalent bond is formed between two atoms when their orbitals overlap and share a pair of electrons. When the orbitals overlap along an axis between the atoms (internuclear axis), they form a sigma bond. In this type of bonding the electron density is highest in the space between the atoms). These covalent bonds have directional properties. This in turn gives carbon the ability to adapt to different molecular and crystal structures. The nature of these bonds outline different physical and chemical properties of carbon allotropes.
Carbon, like most first-row elements in the Periodic Table, has atomic orbitals that can be hybridized (blended). This is due to the fact that the s-orbital and p-orbitals of carbon's second electronic shell energies are very similar. As a result, carbon can be adapted to form chemical bonds in different geometries.
Since carbon dioxide is a covalently bonded molecule it is subjected to three forces that can operate between covalent molecules:
- Dispersion Forces
also known as London Forces (named after Fritz London who first described these forces theoretically 1930) or as van der Waal's Forces - Dipole-dipole interactions
- Hydrogen bonds
Carbon dioxide does not bond with hydrogen (hence the formula CO2) so it is not affected by hydrogen bonds nor is it affected by dipole-dipole interactions because carbon dioxide is a non-polar molecule. So the only intermolecular force that affects it is London dispersion forces:
- momentary dipoles occurring due to uneven electron distributions in neighboring molecules as they approach one another
- the weak residual attraction of the nuclei in one molecule for the electrons in a neighboring molecule.
- The more electrons that are present in the molecule, the stronger the dispersion forces will be.
- Dispersion forces are the only type of intermolecular force operating between non-polar molecules, for example, dispersion forces operate between hydrogen (H2) molecules, chlorine (Cl2) molecules, carbon dioxide (CO2) molecules, dinitrogen tetroxide (N2O4) molecules and methane (CH4) molecules.