Discovery and Production of Dry Ice
Discovery:
Charles Thilorier was a French chemist and scientist who gave the first description of dry ice, or solid carbon dioxide. In 1834, Thilorier opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by rapid evaporation of the liquid product yielded a solid form of carbon dioxide. He is also credited with the discovery of carbon dioxide. Although Thilorier did discover solid carbon dioxide he did not give it the name that we know today. The name was trademarked by the first company to sell dry ice. The Dry Ice Corporation of America first trademarked the name Dry Ice in 1925.
Production:
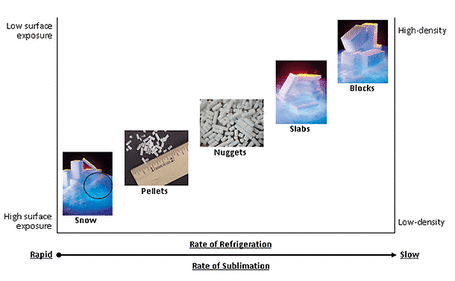
Carbon dioxide used to produce dry ice is a natural byproduct of several industrial manufacturing processes such as fermentation and petrochemical refining. Carbon dioxide emissions from production processes, such as those above, is captured and stored without loss until needed. When dry ice is used, no carbon dioxide is produced. Instead, only the original carbon dioxide released during those manufacturing processes is used which is why dry ice is considered carbon neutral or environmentally friendly. As seen in the above image dry ice can be produced in a variety of forms by control of the rate of refrigeration and sublimation. The main forms are snow, pellets, nuggets, slabs and blocks each with their own specific descriptions, users and applications:
i) Snow: Dry ice snow looks like snow made from the freezing of water and it has a high sublimation rate compared to the pellets, nuggets, slabs and blocks. This form of dry ice requires less complex equipment to achieve, but it has a very short lifespan.
ii) Pellets: Dry ice pellets look like rice and are mainly used for dry ice blasting (more information later). Due to the rate of pellets sublimation being slow and being smaller than dry ice, the use of pellets to freeze food has become very common.
iii) Nuggets: Nuggets of dry ice are typically used for packaging and shipment of food and materials, and are larger than pellets, but smaller than snow.
iv) Slabs: Slices or slabs of dry ice are used predominantly in the field of aviation catering, as a typical slice is 19 mm which fits well into the catering carts. Some companies use the slices for shipment.
v) Blocks: Blocks of dry ice are traditionally used to pack and ship items and keep them cold for a long time. Today, the blocks of dry ice can be used for dry ice blasting. Standard block sizes vary from country to country.
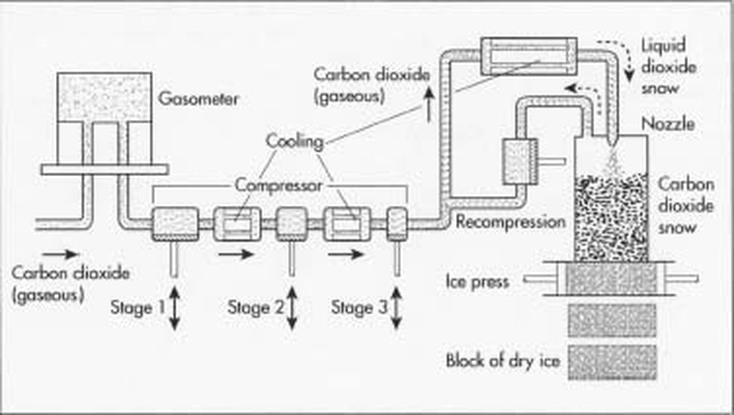
The formation of dry ice is a series of chemical reactions in which liquid carbon dioxide is cooled to below minus 109 degrees Fahrenheit and compressed. The material is injected into a square chamber by a nozzle. It creates dry ice snow and powerful hydraulic cylinders compress the snow into a very dense solid block of dry ice, which weighs 240 pounds. The block is then cut into four slabs, each weighing 60 pounds, for transfer. Dry ice is also formed into nuggets by forcing the snow through a steel die with round holes, which are one-eighth to three-quarters of an inch in diameter. The nuggets are cut at random length of 1-2 inches. The time required depends on the temperature, air movement, and if the dry ice is in a confined space. Dry ice is a expendable refrigerant that leaves nothing behind when it evaporates, so it is desirable for shipment and foods storage.
Charles Thilorier was a French chemist and scientist who gave the first description of dry ice, or solid carbon dioxide. In 1834, Thilorier opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by rapid evaporation of the liquid product yielded a solid form of carbon dioxide. He is also credited with the discovery of carbon dioxide. Although Thilorier did discover solid carbon dioxide he did not give it the name that we know today. The name was trademarked by the first company to sell dry ice. The Dry Ice Corporation of America first trademarked the name Dry Ice in 1925.
Production:
Carbon dioxide used to produce dry ice is a natural byproduct of several industrial manufacturing processes such as fermentation and petrochemical refining. Carbon dioxide emissions from production processes, such as those above, is captured and stored without loss until needed. When dry ice is used, no carbon dioxide is produced. Instead, only the original carbon dioxide released during those manufacturing processes is used which is why dry ice is considered carbon neutral or environmentally friendly. As seen in the above image dry ice can be produced in a variety of forms by control of the rate of refrigeration and sublimation. The main forms are snow, pellets, nuggets, slabs and blocks each with their own specific descriptions, users and applications:
i) Snow: Dry ice snow looks like snow made from the freezing of water and it has a high sublimation rate compared to the pellets, nuggets, slabs and blocks. This form of dry ice requires less complex equipment to achieve, but it has a very short lifespan.
ii) Pellets: Dry ice pellets look like rice and are mainly used for dry ice blasting (more information later). Due to the rate of pellets sublimation being slow and being smaller than dry ice, the use of pellets to freeze food has become very common.
iii) Nuggets: Nuggets of dry ice are typically used for packaging and shipment of food and materials, and are larger than pellets, but smaller than snow.
iv) Slabs: Slices or slabs of dry ice are used predominantly in the field of aviation catering, as a typical slice is 19 mm which fits well into the catering carts. Some companies use the slices for shipment.
v) Blocks: Blocks of dry ice are traditionally used to pack and ship items and keep them cold for a long time. Today, the blocks of dry ice can be used for dry ice blasting. Standard block sizes vary from country to country.
The formation of dry ice is a series of chemical reactions in which liquid carbon dioxide is cooled to below minus 109 degrees Fahrenheit and compressed. The material is injected into a square chamber by a nozzle. It creates dry ice snow and powerful hydraulic cylinders compress the snow into a very dense solid block of dry ice, which weighs 240 pounds. The block is then cut into four slabs, each weighing 60 pounds, for transfer. Dry ice is also formed into nuggets by forcing the snow through a steel die with round holes, which are one-eighth to three-quarters of an inch in diameter. The nuggets are cut at random length of 1-2 inches. The time required depends on the temperature, air movement, and if the dry ice is in a confined space. Dry ice is a expendable refrigerant that leaves nothing behind when it evaporates, so it is desirable for shipment and foods storage.